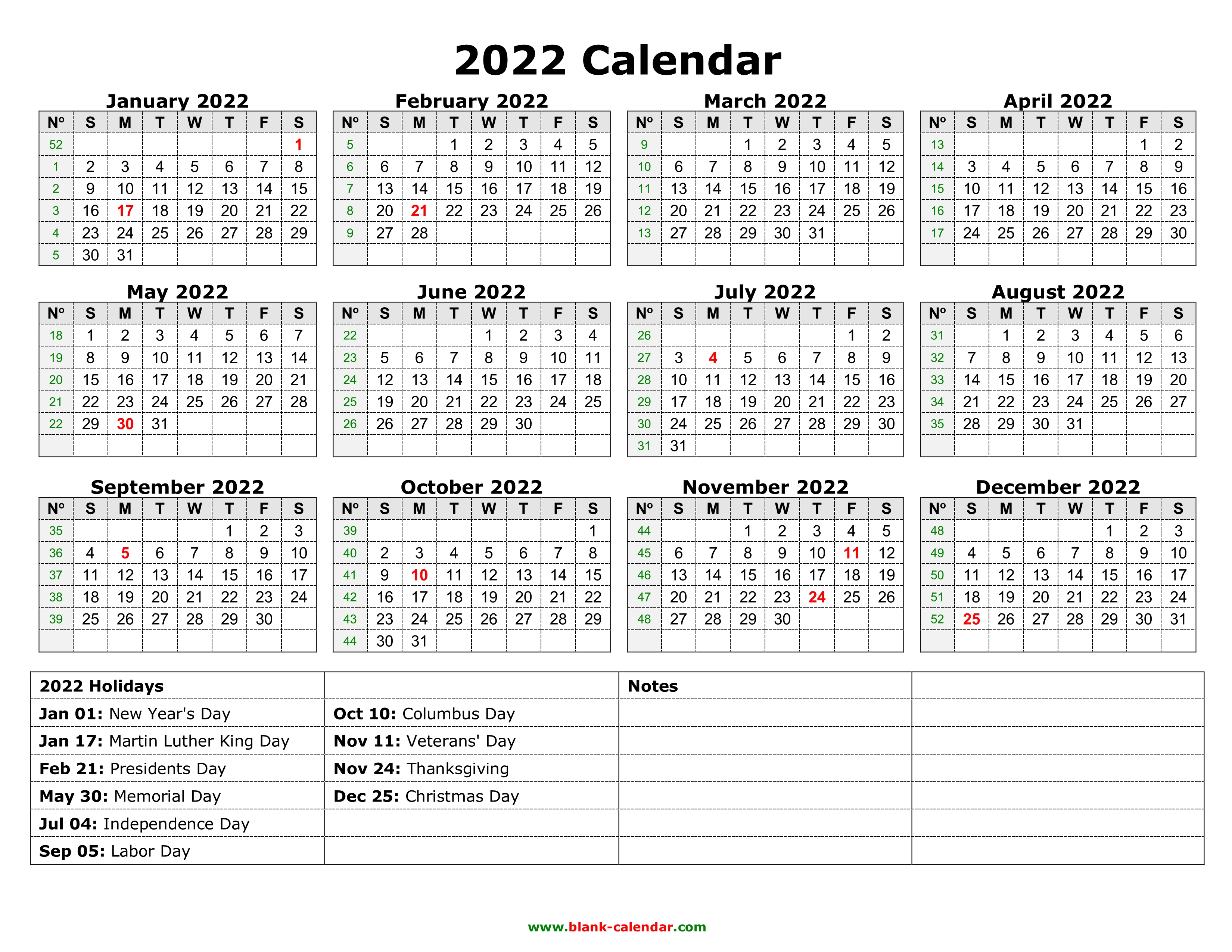
To review the energy levels and. Draw the shapes of s, p, d, and f orbitals. There are five d orbitals, which have more complicated shapes than s and p orbitals. How is it that so many planes are able to fly . An s orbital has a spherical shape and can hold two electrons.
How is it that so many planes are able to fly .
An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. The spdf orbital sets, their shapes, orientations, and spatial overlapping are addressed. Atomic orbitals exactly describe the shape of this atmosphere only when a single electron is present in an atom. Draw the shapes of s, p, d, and f orbitals. To learn about the shapes of the s, p and d orbitals. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Of the four, s and p orbitals are considered because these . Atoms are composed of a heavy nucleus surrounded by light electrons. There are five d orbitals, which have more complicated shapes than s and p orbitals. To review the energy levels and. The 1s is the closest to the nucleus and is smaller . It is a spherical shape. You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the .
Of the four, s and p orbitals are considered because these . Relate the four quantum numbers for an electron to a specific orbital. How is it that so many planes are able to fly . Draw the shapes of s, p, d, and f orbitals. You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the .

When more electrons are added to a single .
The spdf orbital model of the electrons of the . You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the . The behavior of the electrons is governed by the rules of quantum . Of the four, s and p orbitals are considered because these . To learn about the shapes of the s, p and d orbitals. An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. It is a spherical shape. Atomic orbitals exactly describe the shape of this atmosphere only when a single electron is present in an atom. The 1s is the closest to the nucleus and is smaller . The spdf orbital sets, their shapes, orientations, and spatial overlapping are addressed. Atoms are composed of a heavy nucleus surrounded by light electrons. When more electrons are added to a single .
You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the . To learn about the shapes of the s, p and d orbitals. An s orbital has a spherical shape and can hold two electrons. The 1s is the closest to the nucleus and is smaller . The spdf orbital sets, their shapes, orientations, and spatial overlapping are addressed.

Atoms are composed of a heavy nucleus surrounded by light electrons.
To learn about the shapes of the s, p and d orbitals. The 1s is the closest to the nucleus and is smaller . Relate the four quantum numbers for an electron to a specific orbital. Of the four, s and p orbitals are considered because these . Atomic orbitals exactly describe the shape of this atmosphere only when a single electron is present in an atom. Interactive colour surface representations for the five d orbitals in 3d showing the nodes important for transition metal chemistry. The spdf orbital model of the electrons of the . An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. To review the energy levels and. How is it that so many planes are able to fly . Atoms are composed of a heavy nucleus surrounded by light electrons. It is a spherical shape. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape.
50+ Spdf Shapes Gif. You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the . Of the four, s and p orbitals are considered because these . Draw the shapes of s, p, d, and f orbitals. There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. The 1s is the closest to the nucleus and is smaller .