How many electrons can fit on the 2nd level? This video explains s, p, d, and f orbitals, . In writing the electron configuration for sulfur . Electron configuration is a model based on quantum chemistry to show how electrons will be arranged in different orbitals. There are four different orbital shapes:
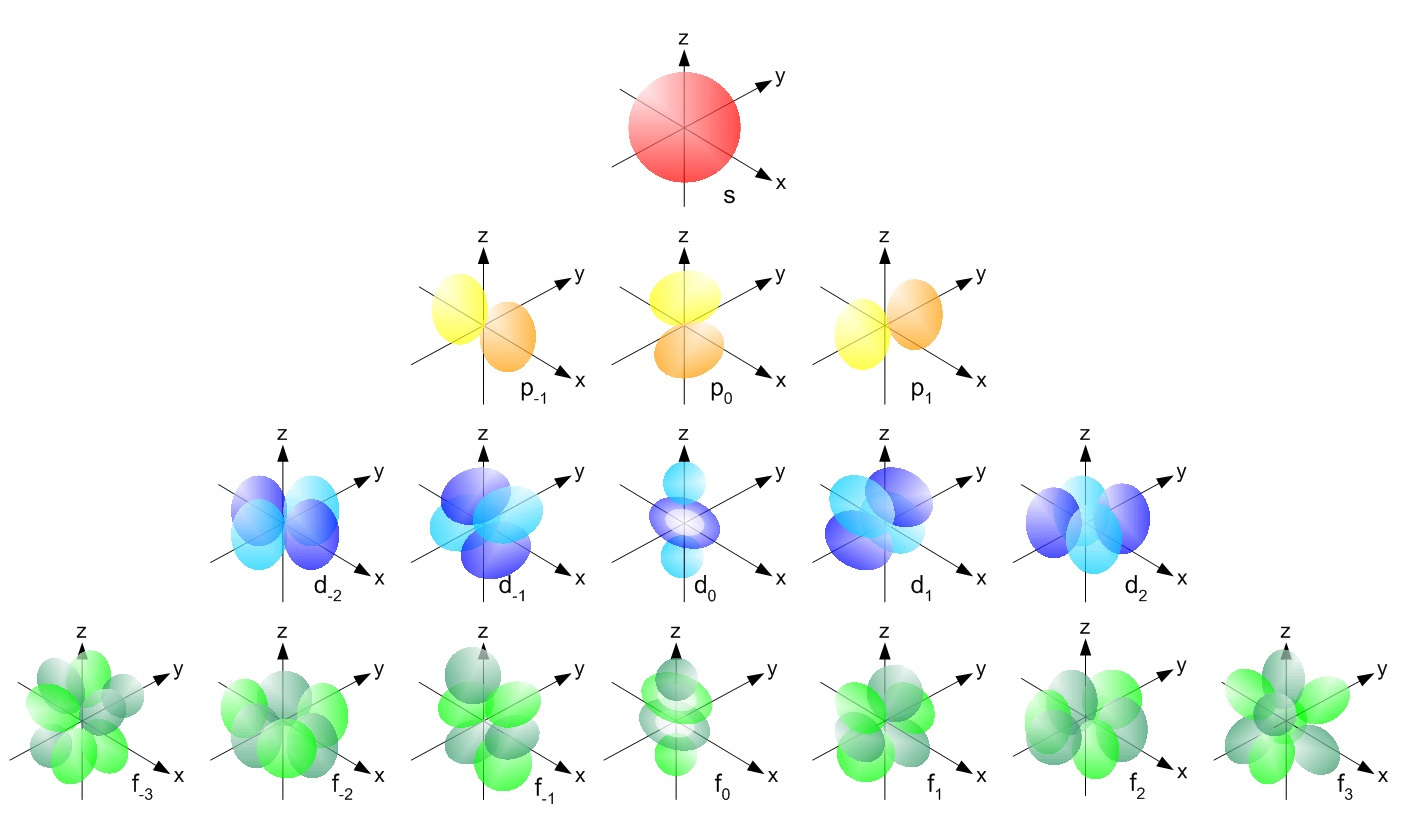
There are four different orbital shapes:
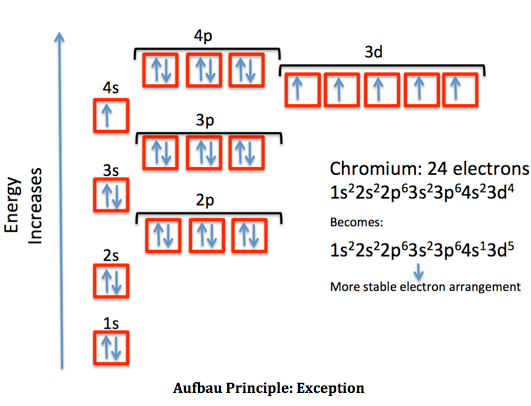
Within each shell, the s . How many electrons can fit on the 2nd level? Electron configuration is a model based on quantum chemistry to show how electrons will be arranged in different orbitals. In a more realistic model, electrons move in atomic orbitals, or subshells. When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the sulfur atom. The periodic table, electron shells, and orbitals. They show up on general chemistry exams without fail. This video explains s, p, d, and f orbitals, . There are four different orbital shapes: S, p, d, and f. Explore the detailed configurations of these orbitals to see . instructor in a previous video, we've introduced ourselves to the idea of an orbital, that electrons don't just orbit a nucleus the way that a planet . In writing the electron configuration for sulfur .
There are four different orbital shapes: Atomic structure and electron configuration. The electron orbitals hold specific numbers of electrons, organized into orbitals of s, p, and d. The periodic table, electron shells, and orbitals. instructor in a previous video, we've introduced ourselves to the idea of an orbital, that electrons don't just orbit a nucleus the way that a planet .

S, p, d, and f.
The electron orbitals hold specific numbers of electrons, organized into orbitals of s, p, and d. instructor in a previous video, we've introduced ourselves to the idea of an orbital, that electrons don't just orbit a nucleus the way that a planet . When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the sulfur atom. They show up on general chemistry exams without fail. In writing the electron configuration for sulfur . There are four different orbital shapes: Explore the detailed configurations of these orbitals to see . This video explains s, p, d, and f orbitals, . S, p, d, and f. Atomic structure and electron configuration. In a more realistic model, electrons move in atomic orbitals, or subshells. How many electrons can fit on the 2nd level? The periodic table, electron shells, and orbitals.
Within each shell, the s . instructor in a previous video, we've introduced ourselves to the idea of an orbital, that electrons don't just orbit a nucleus the way that a planet . Explore the detailed configurations of these orbitals to see . The periodic table, electron shells, and orbitals. They show up on general chemistry exams without fail.
How many electrons can fit on the 2nd level?
In a more realistic model, electrons move in atomic orbitals, or subshells. The electron orbitals hold specific numbers of electrons, organized into orbitals of s, p, and d. This video explains s, p, d, and f orbitals, . Within each shell, the s . How many electrons can fit on the 2nd level? There are four different orbital shapes: S, p, d, and f. Electron configuration tells us how these electrons are distributed among the various atomic orbitals. They show up on general chemistry exams without fail. Electron configuration is a model based on quantum chemistry to show how electrons will be arranged in different orbitals. instructor in a previous video, we've introduced ourselves to the idea of an orbital, that electrons don't just orbit a nucleus the way that a planet . Explore the detailed configurations of these orbitals to see . The periodic table, electron shells, and orbitals.
Download Spdf Orbitals Electron Configuration Gif. In a more realistic model, electrons move in atomic orbitals, or subshells. Atomic structure and electron configuration. Electron configuration tells us how these electrons are distributed among the various atomic orbitals. There are four different orbital shapes: This video explains s, p, d, and f orbitals, .
This video explains s, p, d, and f orbitals, spdf orbitals. In a more realistic model, electrons move in atomic orbitals, or subshells.

