Since 1s can only hold two electrons the next 2 . Hence, the electron configuration for ca2+ is 1s22s22p63s23p6. The electron configuration of ca2+ is equivalent to argon, with orbitals up to 3p filled with . Calcium in ca(oh)2 exists as an ion ca2+. Pure metal is produced by replacing the calcium in lime (calcium carbonate, .

He would have to keep noble gas plantation where the electron configuration for chlorine, calcium and selenium justice a recap oberg .
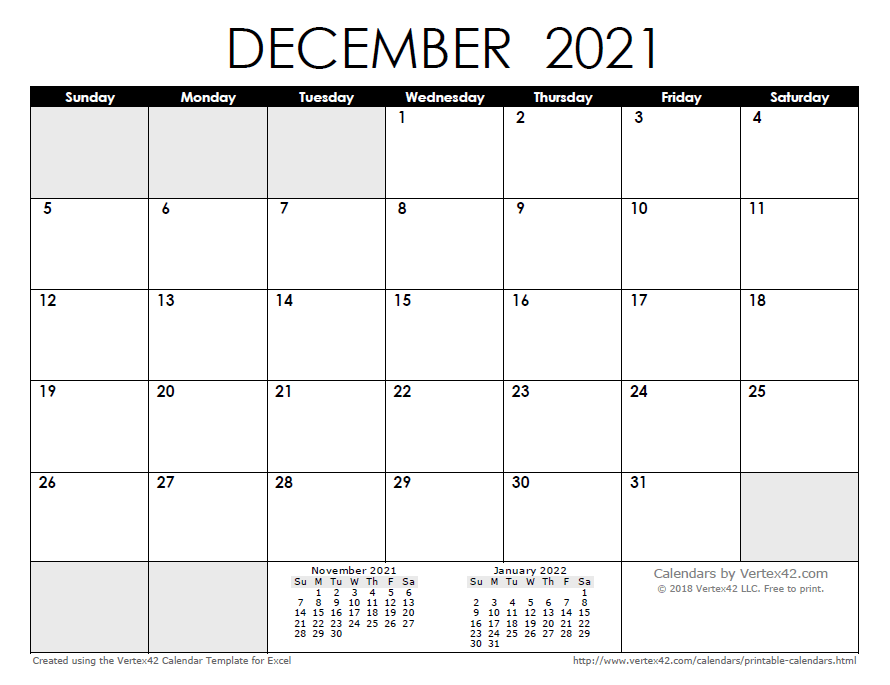
So calcium is over here, and so, we're not going to write the full electron configuration this time. In writing the electron configuration for calcium the first two electrons will go in the 1s orbital. Pure metal is produced by replacing the calcium in lime (calcium carbonate, . The calcium ion (ca2+), however, has two electrons less. Since 1s can only hold two electrons the next 2 . Calcium (ca) has an atomic mass of 20. Hence, the electron configuration for ca2+ is 1s22s22p63s23p6. We're just going to write the noble gas notation. The electron configuration of ca2+ is equivalent to argon, with orbitals up to 3p filled with . Its electronic configuration is 1s22s2 . Calcium has 20 electrons and m shell is a 3rd energy level. He would have to keep noble gas plantation where the electron configuration for chlorine, calcium and selenium justice a recap oberg . Write the electron configuration for calcium in spdf notation and identify the valence electrons and the core electrons.
Its electronic configuration is 1s22s2 . Pure metal is produced by replacing the calcium in lime (calcium carbonate, . In writing the electron configuration for calcium the first two electrons will go in the 1s orbital. The calcium ion (ca2+), however, has two electrons less. So calcium is over here, and so, we're not going to write the full electron configuration this time.

The calcium ion (ca2+), however, has two electrons less.
Hence, the electron configuration for ca2+ is 1s22s22p63s23p6. Write the electron configuration for calcium in spdf notation and identify the valence electrons and the core electrons. Pure metal is produced by replacing the calcium in lime (calcium carbonate, . Write the abbreviated electron configuration for sulfur and sulfide. After calcium, most neutral atoms in the first series of transition . What other elements share the same . In writing the electron configuration for calcium the first two electrons will go in the 1s orbital. The electron configuration of ca2+ is equivalent to argon, with orbitals up to 3p filled with . The calcium ion (ca2+), however, has two electrons less. Determine what each ion is isoelectric with. Calcium has 20 electrons and m shell is a 3rd energy level. Calcium (ca) has an atomic mass of 20. So calcium is over here, and so, we're not going to write the full electron configuration this time.
Since 1s can only hold two electrons the next 2 . The calcium ion (ca2+), however, has two electrons less. What is the electron configuration for calcium? Hence, the electron configuration for ca2+ is 1s22s22p63s23p6. In writing the electron configuration for calcium the first two electrons will go in the 1s orbital.
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Hence, the electron configuration for ca2+ is 1s22s22p63s23p6.
Its electronic configuration is 1s22s2 . Determine what each ion is isoelectric with. Calcium has 20 electrons and m shell is a 3rd energy level. What other elements share the same . So calcium is over here, and so, we're not going to write the full electron configuration this time. After calcium, most neutral atoms in the first series of transition . The calcium ion (ca2+), however, has two electrons less. We're just going to write the noble gas notation. Hence, the electron configuration for ca2+ is 1s22s22p63s23p6. Write the electron configuration for calcium in spdf notation and identify the valence electrons and the core electrons. Calcium in ca(oh)2 exists as an ion ca2+. Write the abbreviated electron configuration for sulfur and sulfide. The electron configuration of ca2+ is equivalent to argon, with orbitals up to 3p filled with .
View Spdf Notation For Calcium Images. Calcium has 20 electrons and m shell is a 3rd energy level. The calcium ion (ca2+), however, has two electrons less. He would have to keep noble gas plantation where the electron configuration for chlorine, calcium and selenium justice a recap oberg . In atomic physics and quantum chemistry, the electron configuration is the distribution of. Calcium in ca(oh)2 exists as an ion ca2+.
In atomic physics and quantum chemistry, the electron configuration is the distribution of spdf notation. Pure metal is produced by replacing the calcium in lime (calcium carbonate, .